Project Description
Definition :
The word “lime” refers to products derived from burnt (calcined) limestone, such as quicklime and hydrated lime. Limestone is a naturally occurring and abundant sedimentary rock consisting of high levels of calcium and/or magnesium carbonate, and/or dolomite (calcium and magnesium carbonate), along with small amounts of other minerals. It is extracted from quarries and underground mines all over the world.
Click to learn about the material and purchase caustic soda flake
FEATURES
lime is used :
- In our homes for copper, dry bleaches, dyes, glass, gold, lawn and garden neutralizers, leather, masonry, mortar, paint, paper, pharmaceuticals, plaster and stucco, sugar, table salt, toothpaste and tortilla flour.
- In our communities for agricultural liming, apple storage, asphalt, sewage treatment and water treatment.
- For the preservation of our environment in mine tailing drainage treatment, air pollutant abatement, epidemic diseases control, remediation, waste water treatment.
- In industries for alumina, barium, glass, lead, lithium, magnesium, nickel, petroleum refining, silver, sodium alkali, solar-grade silica, steel, uranium, wood pulp, zinc.
THE LIME CYCLE
After processing, products derived from limestone have the unique ability to return to their original chemical form. The lime cycle consists of first burning of limestone to form quicklime. Hydrated lime can then be produced by adding water to the quicklime. At this point, carbon dioxide in the atmosphere or from industrial processes react with hydrated lime to convert it back to limestone. This cycle is called the lime cycle. The time it takes for quicklime or hydrated lime to be converted back to limestone can span from less than an hour with the aid of certain industrial processes to several years if left at atmospheric conditions.
Details on raw material
Calcium oxide (also called quicklime, burnt lime, or unslaked lime) is formed by burning limestone. Quicklime reacts with water, generating a great amount of heat. We distinguish soft-burnt lime, medium-burnt lime, and hard-burnt lime. In the construction industry, quicklime is added to mortar. It can also be used as a drying or neutralizing agent, as fertilizer lime and for the production of lime mortar and lime plaster.
By adding water, calcium oxide is transformed into calcium hydroxide (also called lime hydrate, hydrated lime or slaked lime). Lime hydrate can, for example, be used instead of limestone for flue gas desulfurization. To be noted that the amount of lime hydrate is smaller than that of limestone. Derived from quicklime, gypsum (calcium sulfate) has a whiteness of about 80% and is ready for other uses.
Packing :
1- Jumbo bags
2- 2ply 25 and 50 Kg bags
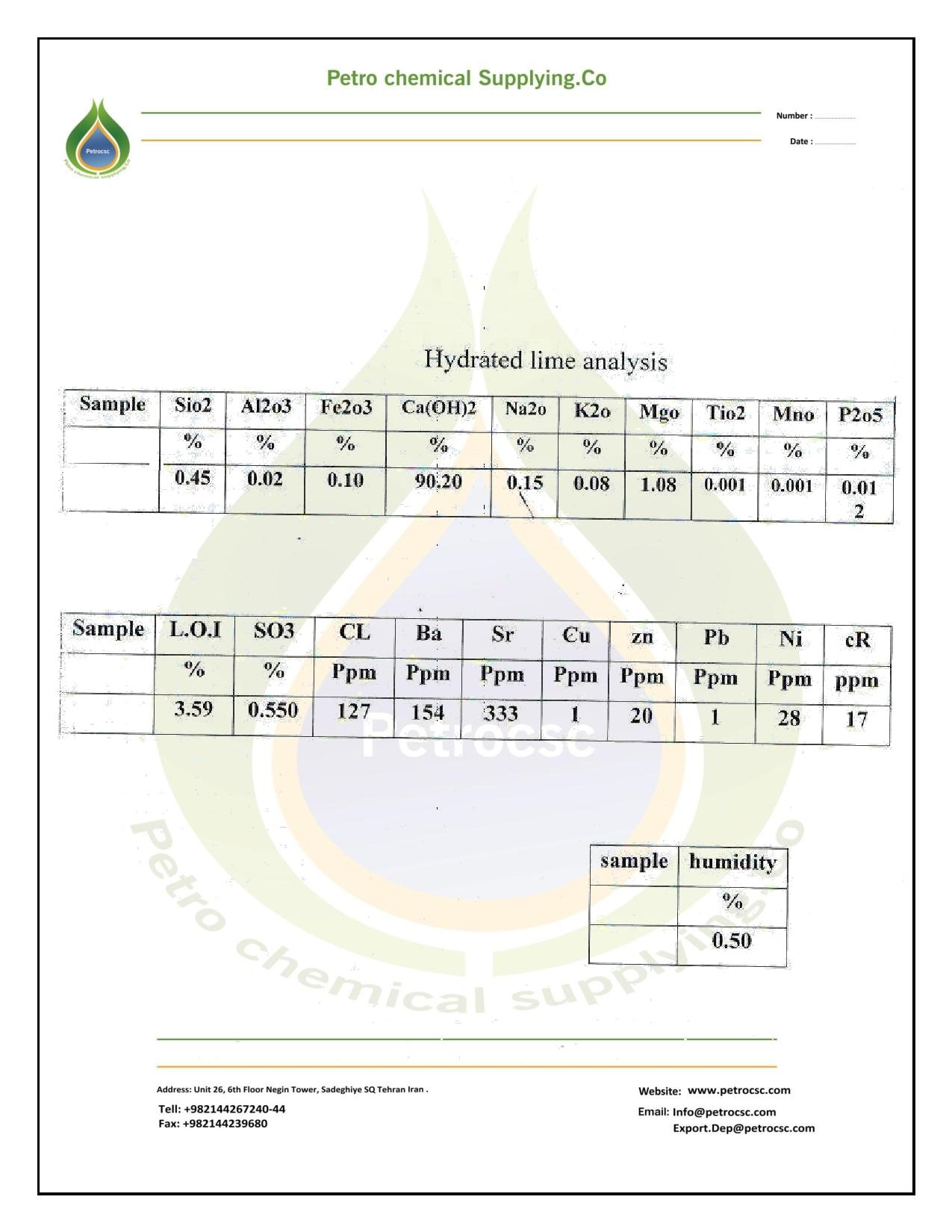
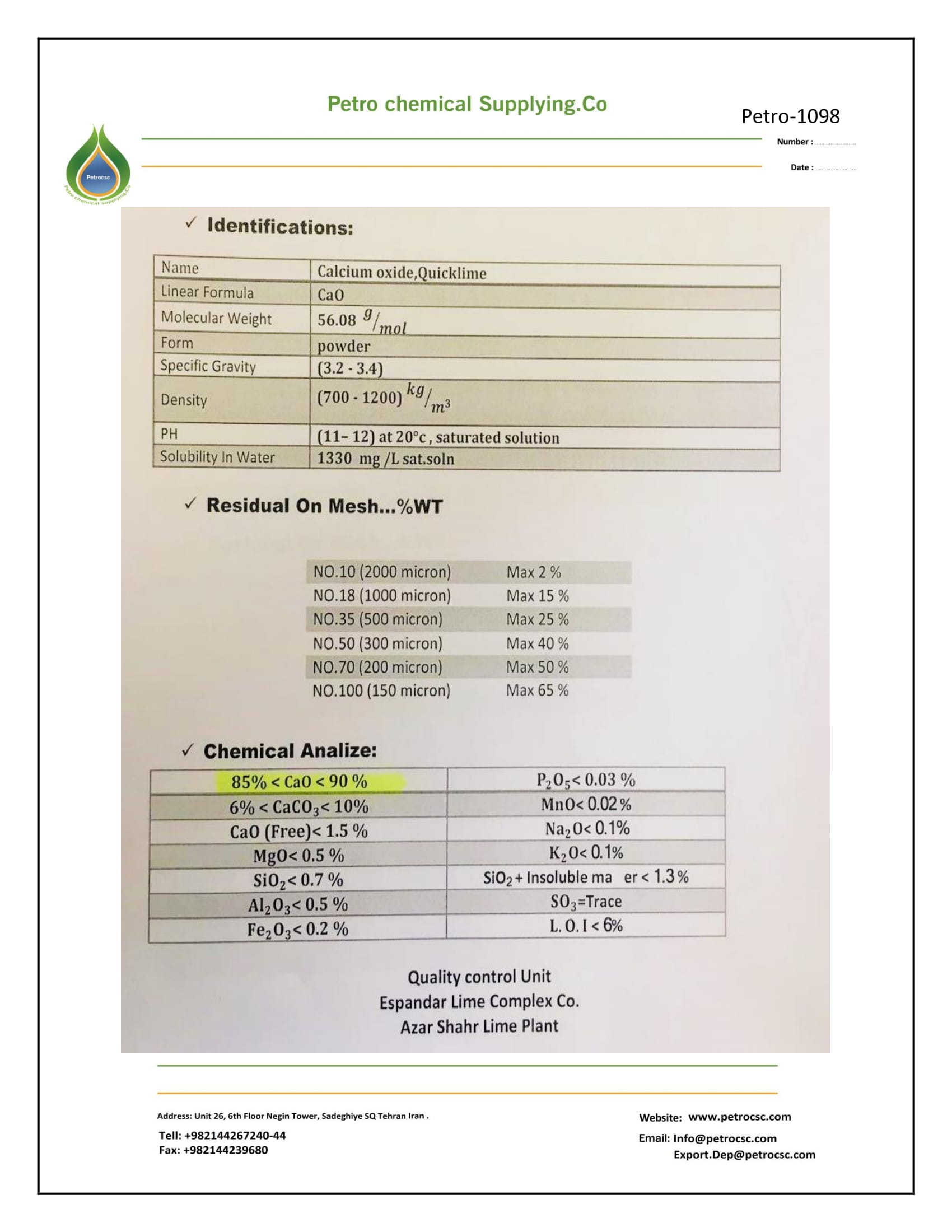
SPECIFICATION